For decades, the blood-brain barrier (BBB) has stood as a formidable gatekeeper, selectively shielding the brain from harmful substances while frustrating efforts to deliver life-saving therapeutics. This biological fortress, while essential for protecting our most vital organ, has rendered many promising treatments for neurological disorders ineffective. Now, a groundbreaking approach combining protein engineering and artificial intelligence is cracking the code to safe BBB penetration, potentially revolutionizing treatment for Alzheimer's, Parkinson's, and brain cancers.
The Blood-Brain Barrier Conundrum
Nature's most selective filter, the BBB, is composed of tightly packed endothelial cells lining cerebral blood vessels. These cells form continuous tight junctions that prevent approximately 98% of small-molecule drugs and nearly 100% of large-molecule therapeutics from reaching brain tissue. While this protection is crucial for maintaining the brain's delicate chemical balance, it creates an enormous challenge for treating neurological conditions. Traditional approaches to bypass the BBB—such as direct injection or temporary barrier disruption—carry significant risks including infection, neurotoxicity, and tissue damage.
Researchers have long sought to exploit natural BBB transport mechanisms, particularly receptor-mediated transcytosis (RMT). This biological process allows certain proteins to ferry essential nutrients across the barrier by binding to specific receptors on the endothelial cell surface. The challenge has been designing synthetic carriers that can hijack these pathways without triggering immune responses or causing unintended side effects.
Protein Keys Meet AI Locksmiths
The recent breakthrough comes from an unexpected marriage of structural biology and machine learning. Scientists have developed computational methods to design protein-based "smart keys" that perfectly fit the biological locks (receptors) on the BBB surface. These engineered proteins, when assembled into nanocarriers, can trick the brain's defense system into granting them passage.
At the heart of this innovation lies deep learning algorithms trained on thousands of protein-receptor interactions. These AI systems can predict how subtle modifications to protein structures will affect their binding affinity and transport efficiency across the BBB. Unlike traditional trial-and-error approaches, the AI can explore millions of potential configurations virtually before any physical experiments begin.
Nanoscale Engineering Marvels
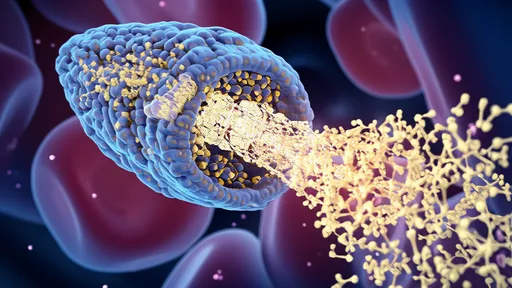
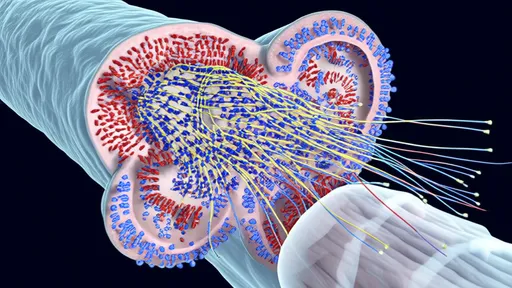
The resulting nanocarriers represent a masterpiece of biological engineering. Measuring just 20-100 nanometers in diameter—about 1/1000th the width of a human hair—these spherical vehicles combine several clever design features. Their outer shells display multiple copies of the engineered protein "keys" that engage with BBB receptors. The interior can be loaded with diverse therapeutic cargo, from small-molecule drugs to gene-editing tools.
Perhaps most impressively, these carriers incorporate feedback mechanisms that ensure they release their payload only after successful BBB transit. Some versions use the slightly more acidic environment of brain tissue as their trigger, while others respond to specific enzymes present in the neural extracellular space. This controlled release prevents the drugs from being ejected back into the bloodstream during the transcytosis process.
From Concept to Preclinical Success
Early results from animal studies have been striking. In models of glioblastoma, the most aggressive form of brain cancer, AI-designed nanocarriers delivered chemotherapy drugs with 15-fold greater brain accumulation compared to conventional administration. Even more remarkably, the targeted approach reduced off-target toxicity, allowing for higher effective doses without the usual systemic side effects.
For neurodegenerative diseases, researchers have demonstrated successful delivery of antibody therapies across the BBB—a feat previously thought impossible without invasive methods. In Alzheimer's models, nanocarriers transporting beta-amyloid targeting antibodies showed significant reduction in plaque burden after just four weeks of treatment.
Overcoming Historical Hurdles
Previous attempts at BBB penetration have faltered for several reasons. Antibody-based approaches often suffered from poor penetration rates, requiring impractically large doses. Viral vectors raised safety concerns about insertional mutagenesis and immune reactions. Non-targeted nanoparticles tended to accumulate in the liver and spleen rather than reaching the brain.
The new generation of AI-designed carriers addresses these limitations through their precision engineering. Their protein components are humanized to minimize immune detection, and their small size avoids rapid clearance by the reticuloendothelial system. Perhaps most crucially, their receptor targeting is highly selective—binding only to BBB-specific transport receptors rather than ubiquitous cell surface markers.
The Road to Clinical Translation
While the technology shows immense promise, significant challenges remain before clinical application. Scaling up production of these complex nanostructures while maintaining batch-to-batch consistency presents engineering hurdles. Regulatory agencies will require extensive safety data on long-term effects of repeated BBB penetration. Researchers must also demonstrate that the approach works equally well in aged brains, where the BBB's properties may differ from young, healthy models.
Several biotechnology companies have already licensed the core AI platforms and are advancing lead candidates toward Investigational New Drug (IND) applications. Industry observers predict the first human trials for brain cancer applications could begin within 2-3 years, with neurodegenerative disease programs following shortly after.
A New Era in Neurological Medicine
The implications extend far beyond current treatment paradigms. Successfully shuttling drugs across the BBB opens possibilities for: precise gene editing in neurological disorders, targeted delivery of neuroprotective factors after stroke or trauma, and even the reversal of age-related cognitive decline. The technology may also enable new diagnostic approaches, allowing imaging contrast agents to highlight early pathological changes currently invisible to conventional scans.
As the field progresses, researchers envision increasingly sophisticated nanocarriers capable of delivering multiple therapeutic agents in sequence or responding to disease biomarkers to release their payload only when and where needed. Some teams are working on "smart" systems that could potentially navigate to specific brain regions based on unique receptor patterns.
Ethical Considerations and Future Directions
With such powerful technology comes necessary ethical scrutiny. The ability to circumvent the BBB raises questions about potential misuse, from unauthorized cognitive enhancement to malicious delivery of neuroactive substances. The scientific community emphasizes the importance of establishing robust ethical frameworks alongside technical development.
Looking ahead, the convergence of AI and nanotechnology appears poised to transform our approach to brain disorders. What began as an attempt to sneak drugs past a biological barrier may ultimately provide the key to understanding and treating some of medicine's most challenging conditions. As one lead researcher remarked, "We're not just delivering drugs—we're delivering hope across the final frontier of human physiology."
For decades, the blood-brain barrier (BBB) has stood as a formidable gatekeeper, selectively shielding the brain from harmful substances while frustrating efforts to deliver life-saving therapeutics. This biological fortress, while essential for protecting our most vital organ, has rendered many promising treatments for neurological disorders ineffective. Now, a groundbreaking approach combining protein engineering and artificial intelligence is cracking the code to safe BBB penetration, potentially revolutionizing treatment for Alzheimer's, Parkinson's, and brain cancers.
In a groundbreaking development that could revolutionize the treatment of spinal cord injuries, researchers have successfully engineered hydrogel-based optical fibers capable of mimicking neural pathways. These "neural optical fibers" represent a fusion of advanced materials science and neurobiology, offering new hope for patients with previously untreatable damage to their central nervous system.
In a groundbreaking discovery that could revolutionize our approach to plastic waste, scientists have identified a surprising ally in the fight against polyethylene pollution: the humble wax worm. More specifically, the bacteria residing in the gut of these caterpillar-like creatures have demonstrated an extraordinary ability to break down one of the world's most stubborn plastics. This finding opens new avenues for tackling the global plastic crisis through biological means.
In a groundbreaking development that challenges our understanding of aging, scientists have demonstrated the potential to reverse cellular aging through a technique called transient reprogramming. This approach temporarily rewinds the epigenetic "clock" of cells without erasing their identity, opening new possibilities for regenerative medicine and age-related disease treatment.
In a groundbreaking leap for precision medicine, researchers have unveiled a light-controlled CRISPR delivery system using DNA-origami "nanodrones" – a fusion of nanotechnology and gene editing that promises unprecedented control over therapeutic targeting. This innovation, emerging from a collaboration between bioengineers and geneticists, reimagines drug delivery by combining the programmability of DNA nanostructures with the spatial precision of optogenetics.
In a groundbreaking study that could reshape our understanding of consciousness, neuroscientists have identified the thalamic reticular nucleus (TRN) as a potential "rhythmic switch" governing wakefulness through gamma wave modulation. This almond-shaped inhibitory structure, often described as the brain's gatekeeper, appears to orchestrate states of awareness by tuning neural oscillations like a conductor leading a symphony of consciousness.
For decades, chronic pain has remained one of medicine's most elusive challenges – a complex interplay of biological, psychological, and social factors that often defies conventional treatment. Now, groundbreaking research into the spinal cord's neural "fingerprints" of pain is revolutionizing our understanding of how persistent pain becomes etched into the nervous system. Scientists are mapping specialized neural circuits that appear to encode chronic pain with remarkable specificity, opening new avenues for targeted therapies.
In a groundbreaking discovery that reshapes our understanding of brain metabolism, researchers have identified glial cells as the unsung architects of neuronal energy distribution. The study reveals how these long-overlooked support cells orchestrate the precise mitochondrial allocation to neurons, challenging the neuron-centric dogma of neuroscience. This paradigm shift underscores glial cells' role as metabolic conductors in the symphony of brain function.
Scientists have uncovered a startling new pathway by which gut microbes communicate with the brain at lightning speed. Dubbed the "10-second gut-brain superhighway," this discovery centers on the vagus nerve's ability to transmit microbial signals faster than previously thought possible. The findings could revolutionize our understanding of conditions ranging from depression to irritable bowel syndrome.
In a groundbreaking discovery that bridges molecular biology and neuroscience, researchers have uncovered how the CPEB protein acts as a "molecular glue" to solidify long-term memories through an elegant phase transition mechanism. This finding not only revolutionizes our understanding of memory persistence but also reveals nature's ingenious solution to maintaining information at the molecular level.
In a groundbreaking discovery that blurs the line between botany and acoustics, researchers have uncovered evidence of tomatoes employing ultrasonic warfare against herbivorous insects. The study, published in Nature Plants, reveals how tomato plants emit high-frequency sounds when under attack - not as passive victims, but as active participants in their own defense.
In the dense rainforests of Central and South America, leafcutter ants have perfected an architectural marvel that puts human climate control systems to shame. These tiny engineers construct elaborate underground nests spanning hundreds of square feet, maintaining near-perfect temperature and humidity levels year-round – without using a single watt of electricity. As architects and engineers grapple with the urgent need to reduce building emissions, these insect-built structures offer profound lessons in passive climate regulation.
The depths of the ocean hold secrets that continue to astonish scientists, and among the most enigmatic phenomena is the "whale fall"—the carcass of a deceased whale sinking to the seabed. These massive organic deposits create transient ecosystems, supporting diverse marine life for decades. But beyond the visible scavengers and bone-eating worms, a hidden microbial world thrives, and within it, something extraordinary has been discovered: colossal bacteriophages, viruses that prey on bacteria, with genomes so large they defy conventional understanding.
In a groundbreaking discovery that could revolutionize coral reef monitoring, scientists have identified a natural early warning system for coral bleaching events. Certain coral species exhibit vivid fluorescent patterns when under thermal stress, acting as biological "sentry lights" that signal the onset of bleaching before visible damage occurs. This phenomenon, observed in reef-building corals across the Indo-Pacific, represents nature's own climate change alert system.
In a groundbreaking development for ecological research and climate science, researchers have harnessed advanced LiDAR technology to map the photosynthetic efficiency of trees across vast forested areas. Dubbed the "carbon pulse" of forests, this innovative approach provides unprecedented insights into how trees absorb and process carbon dioxide at an ecosystem scale. The implications for understanding carbon sequestration, forest health monitoring, and climate change mitigation strategies are profound.